Healthcare Medical Devices Biotechnology

Medical Engineered Materials Market

Medical Engineered Materials Market Size, Share, Growth & Industry Analysis, By Type (Plastics, Foams, Films, Adhesives, Elastomers), By Application (Medical Devices, Medical Disposable, Medical Wearable, Advanced Woundcare) and Regional Analysis, 2024-2031
Pages : 140
Base Year : 2023
Release : March 2025
Report ID: KR1426
Market Definition
The medical engineered materials market encompasses the development, manufacturing, and supply of specialized materials tailored for medical and healthcare applications. These materials, including high-performance polymers, elastomers, foams, hydrogels, silicones, and advanced composites, are engineered to meet stringent medical standards for biocompatibility, durability, sterilizability, and performance.
Medical Engineered Materials Market Overview
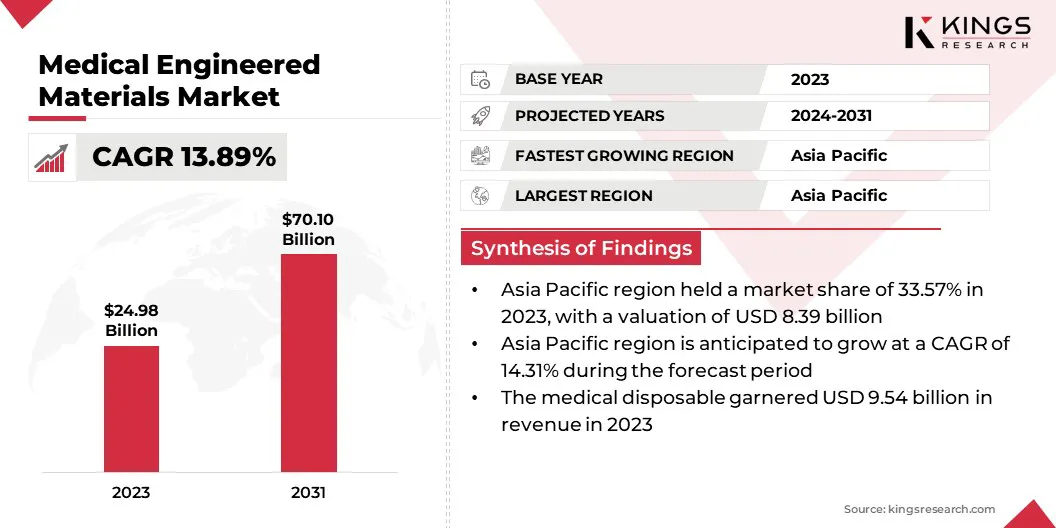
The global medical engineered materials market size was valued at USD 24.98 billion in 2023 and is projected to grow from USD 28.21 billion in 2024 to USD 70.10 billion by 2031, exhibiting a CAGR of 13.89% during the forecast period.
This significant market expansion is driven by the increasing demand for advanced materials in medical applications, including medical devices, implants, drug delivery systems, and diagnostic equipment.
Furthermore, the rising prevalence of chronic diseases, advancements in healthcare infrastructure, and the growing adoption of minimally invasive surgical procedures are key factors fueling this growth.
Major companies operating in the global medical engineered materials Industry are SABIC, Teknor Apex, Covestro AG, W. L. Gore & Associates, Inc., Celanese Corporation, Solvay, Johnson & Johnson Private Limited, Nitto Denko Corporation, Evonik Industries AG, Eastman Chemical Company, Trelleborg Group, B. Braun SE, BASF, DuPont de Nemours, Inc., DSM, and others.
Furthermore, increasing government and private sector investments in healthcare, particularly in emerging markets, are expanding the application scope of medical engineered materials. The shift toward sustainable and biodegradable materials, supported by environmental concerns and regulatory policies, is further shaping market landscape.
Key Highlights:
- The global medical engineered materials market size was recorded at USD 24.98 billion in 2023.
- The market is projected to grow at a CAGR of 13.89% from 2024 to 2031.
- Asia Pacific held a share of 33.57% in 2023, valued at USD 8.39 billion.
- The plastics segment garnered USD 7.93 billion in revenue in 2023.
- The medical disposable segment is expected to reach USD 23.57 billion by 2031.
- North America is anticipated to grow at a CAGR of 13.99% over the forecast period.
Market Driver
"Growing Demand for Advanced Medical Devices"
The medical engineered materials market is witnessing substantial growth due to the increasing demand for advanced medical devices and equipment. The rising prevalence of chronic diseases such as cardiovascular disorders, diabetes, and orthopedic conditions has highlighted the rising need for innovative medical solutions, including implants, prosthetics, and drug delivery systems.
Additionally, the aging global population is boosting demand for biocompatible and durable materials that enhance patient outcomes and improve the performance of medical devices.
Technological advancements in material science, such as the development of high-performance polymers, elastomers, and bioresorbable materials, are further propelling market expansion. Medical engineered materials enable enhanced durability, flexibility, and antimicrobial properties, making them ideal for critical healthcare applications.
- According to United Nations population projections, between 1974 and 2024, the global population aged 65 and above increased from 5.5% in 1974 to 10.3% in 2024. PThis trend is expected to continue, with the senior population expected to reach 20.7%, significantly impacting demographic and economic dynamics.
Market Challenge
"Regulatory Compliance and Certification"
A key challenge impeding the expansion of the medical engineered materials market is the stringent regulatory requirements governing their development and commercialization.
Authorities such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and other global health agencies enforce strict standards to ensure biocompatibility, safety, and performance. Compliance with these regulations often results in prolonged approval timelines, increased research costs, and complex certification processes for manufacturers.
To overcome this challenge, companies must invest in proactive regulatory strategy development. Early collaboration with regulatory bodies, third-party testing labs, and compliance experts can help streamline the approval process.
Moreover, implementing advanced material testing methodologies, conducting extensive clinical evaluations, and maintaining transparent documentation can enhance compliance efficiency.
Market Trend
"Adoption of Sustainable and Biodegradable Materials"
A prominent trend shaping the medical engineered materials market is the increasing adoption of sustainable and biodegradable materials in medical applications. Growing environmental concerns, coupled with regulatory pressures to reduce medical waste, are prompting manufacturers to develop eco-friendly alternatives to traditional synthetic materials.
Biodegradable polymers, bio-based elastomers, and plant-derived hydrogels are increasingly used in medical implants, drug delivery systems, and wound care products. This shift is particularly evident in the development of bioresorbable materials, which naturally degrade within the body over time, reducing long-term complications.
Additionally, advancements in green chemistry and biopolymer processing are enabling the production of high-performance materials with improved sustainability and reduced carbon footprint.
- In December 2024, MIT chemical engineers developed an eco-friendly alternative to traditional microbeads, advancing the market. These biodegradable particles enable improved drug delivery and nutrient encapsulation, fostering innovation in medical coatings, supplements, and wound care while aligning with sustainability trends and evolving regulatory standards.
Medical Engineered Materials Market Report Snapshot
|
Segmentation |
Details |
|
By Type |
Plastics, Foams, Films, Adhesives, Elastomers |
|
By Application |
Medical Devices (Diagnostic Equipment, Surgical Equipment, Dental Tools, Others), Medical Disposable (Surgical Instrument & Supplies, Diagnostic & Laboratory Disposables, Medical & Laboratory Gloves, Others) , Medical Wearable (Smart Watch, Activity Monitor, Patches, Others), Advanced Woundcare (Dressings, Devices & Accessories, Graft & Matrices, Others). |
|
By Region |
North America: U.S., Canada, Mexico |
|
Europe: France, U.K., Spain, Germany, Italy, Russia, Rest of Europe |
|
|
Asia-Pacific: China, Japan, India, Australia, ASEAN, South Korea, Rest of Asia-Pacific |
|
|
Middle East & Africa: Turkey, UAE, Saudi Arabia, South Africa, Rest of Middle East & Africa |
|
|
South America: Brazil, Argentina, Rest of South America |
Market Segmentation
- By Type (Plastics, Foams, Films, Adhesives, and Elastomers): The plastics segment earned USD 7.93 billion in 2023 due to its widespread adoption in medical device manufacturing, superior biocompatibility, and versatility in applications such as implants, surgical instruments, and drug delivery systems.
- By Application (Medical Devices, Medical Disposable, Medical Wearable, and Advanced Woundcare): The medical disposable held a share of 33.84% in 2023, mainly propelled by the rising demand for single-use medical products that minimize the risk of infections and cross-contamination.
Medical Engineered Materials Market Regional Analysis
Based on region, the global market has been classified into North America, Europe, Asia Pacific, Middle East & Africa, and Latin America.
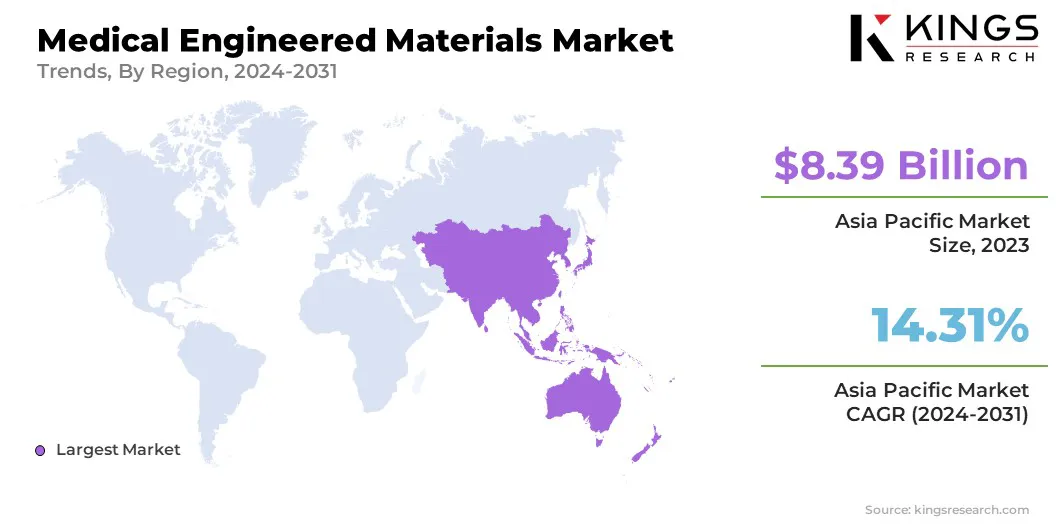
The Asia Pacific medical engineered materials market accounted for for notable share ofd around 33.57% in 2023, valued at USD 8.39 billion. This dominance is attributed to rapid industrialization, increasing healthcare expenditure, and a growing demand for advanced medical devices in countries such as China, India, and Japan.
The expansion of the region’s medical manufacturing sector, coupled with rising investments in research and development, is fueling regional market growth. Additionally, government initiatives to improve healthcare accessibility and the increasing presence of global medical device manufacturers are fostering the adoption of medical engineered materials in the region.
North America medical engineered materials Industry is poised to grow at a significant CAGR of 13.99% over the forecast period. This growth is bolstered by strong healthcare infrastructure, high R&D investments, and early adoption of innovative medical technologies.
The presence of leading medical device manufacturers, stringent regulatory frameworks, and increasing demand for high-performance biocompatible materials are further contributing to thist growth.
The rising prevalence of chronic diseases and an aging population are boosting demand for advanced medical engineered materials in applications such as implants, drug delivery systems, and diagnostic devices.
Regulatory Frameworks
- In the U.S., the Food and Drug Administration (FDA) regulates the medical engineered materials market, ensuring safety, biocompatibility, and performance in medical devices, implants, and drug delivery systems.
- In Europe, the European Medicines Agency (EMA) and the European Commission (EC) oversees the market under MDR, IVDR, and REACH frameworks. These regulations ensure material safety, performance, and compliance, with Notified Bodies (NBs) conducting conformity assessments before market entry.
Competitive Landscape
The global medical engineered materials market is characterized by a large number of participants, including both established corporations and emerging players. Leading companies are focusing on strategic partnerships, mergers & acquisitions, and research & development to enhance their product portfolios and strengthen their market presence.
They are further investing in advanced material technologies, including biocompatible polymers, antimicrobial coatings, and bioresorbable materials, to meet the evolving demands of the healthcare sector.
Established corporations leverage their extensive distribution networks, regulatory expertise, and strong financial capabilities to maintain a competitive edge. Meanwhile, emerging organizations and specialized material developers drive market disruption by introducing high-performance and sustainable medical materials for next-generation medical devices.
The market is set to witness sustained growth, with companies striving to enhance functionality, safety, and sustainability to meet increasing global demand.
List of Key Companies in Medical Engineered Materials Market:
- SABIC
- Teknor Apex
- Covestro AG
- W. L. Gore & Associates, Inc.
- Celanese Corporation
- Solvay
- Johnson & Johnson Private Limited
- Nitto Denko Corporation
- Evonik Industries AG
- Eastman Chemical Company
- Trelleborg Group
- B. Braun SE
- BASF
- DuPont de Nemours, Inc.
- DSM
Recent Developments (New Product Launch)
- In April 2024, AES Clean Technology introduced CleanLock, a modular cleanroom solution designed to enhance cleanliness and operational efficiency. This innovation is boosting demand for high-performance, contamination-resistant materials in the medical engineered materials market, aligning with stringent regulatory requirements in medical device manufacturing and pharmaceutical production.
CHOOSE LICENCE TYPE
Frequently Asked Questions (FAQ's)
Get the latest!
Get actionable strategies to empower your business and market domination
- Deliver Revenue Impact
- Demand Supply Patterns
- Market Estimation
- Real-Time Insights
- Market Intelligence
- Lucrative Growth Opportunities
- Micro & Macro Economic Factors
- Futuristic Market Solutions
- Revenue-Driven Results
- Innovative Thought Leadership
.webp)